What is Dry Ether| Preparation | Properties | Application
What Is Dry Ether, and Why Should We Care?
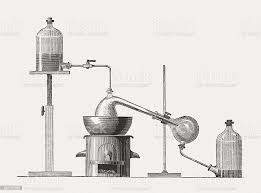
Dry Ether
The main advantage of Dry Ether is that it can be used in a wide range of chemistry processes. Because ether is highly flammable, it is a safety hazard. In an organic chemistry laboratory, open flames are not permitted. In a lab environment, it is vital to use proper ventilation. For this reason, there are a number of safety precautions that can be taken when using a dry ether.
Dry ether is a volatile and flammable organic liquid. It is commonly used in laboratory settings and as a starter fluid for cars. Historically, ether was used as a general anesthetic. In modern times, it is used in the manufacture of plastics and in the production of cellulose. The benefits of Dry Ether for chemical synthesis are many. It is one of the most versatile organic solvents in the world, with applications in a wide range of fields.
Dry ether is extremely flammable. Any vaporized ether will catch fire, and if water is nearby, the fire will spread. A damp cloth or sand should be used to put out the hazard. The fumes of ethanol can be anesthetic. A prolonged exposure can leave a person dizzy or drowsy. It is important to always remember the risks associated with the use of a solvent like ethyl ether.
Dry ether is an aprotic solvent. It has a large therapeutic/safety window and can be used in a variety of chemical reactions. It is an excellent choice for laboratory purposes and is used in the preparation of Grignard reagents. Its aprotic nature is also a major benefit when performing a reaction involving an alkyl halide. It can also be used as a starting fluid in cold climates.
As a solvent, Dry Ether is a popular alternative to benzene. It is a flammable solvent and is the second most common chemical in the world. It is used for a variety of purposes, and can even be used for anesthesia. While it is an excellent alternative to aqueous anesthetic, it has many downsides. It is a polar liquid. A polar solvent has more a negative polarity than an aqueous solution.
The positive azeotrope phase diagram shows the boiling point of a liquid at point A. When a mixture reaches this temperature, it becomes vapor. When the vapor is at equilibrium, it is richer in constituent X than the liquid. As the process progresses, it gradually becomes a mixture of both X and Y. In the case of dry ether, the vapor of composition B is collected over the liquid of the opposite composition A.
An azeotrope is a chemical that has a constant boiling point. As a result, the proportions of the mixture remain the same even after distillation. Azeotropes are also known as constant boiling point mixtures. This means that they have the same constituents as the unboiled liquid. This makes them ideal for distillation. It is also a good solvent for cleaning purposes. Soluble ether can be found in many products.
For amazing Technology Blogs visit - THE TECHJOURNAL
For details on What is mobile edge computing visit - Tech Journal


Comments
Post a Comment